- How Do Atoms Form Compounds?
- How do atoms make compounds?
- Why do atoms form compounds?
- How are compounds formed?
- What kind of atoms form compounds?
- Does atoms combine to form compounds?
- How do atoms bond with each other to form molecules?
- Why do atoms form ionic bonds?
- How are compounds formed and separated?
- What creates an ionic compound?
- How are compounds formed 8?
- Why do atoms form compounds quizlet?
- When elements react their atoms join with other atoms what type of substance is formed?
- What will be produced when two different atoms are combined?
- When and where were the first compounds created?
- How does an atom become a positive ion?
- How are covalent compounds formed?
- What is formed after an atom gains electrons?
- How are compounds formed short answer?
- What are the 10 compounds?
- What are the two parts of an atom What are they made up of?
- How do atoms form chemical bonds quizlet?
- How is an ionic bond formed quizlet?
- How are combinations of different atoms held together?
- How atoms unite and change into molecules?
- Can atoms be divided or broken?
- What is formed when two or more atoms chemically combine?
- How were the first compounds created?
- What is the first compound formed?
- What was the first atom formed?
- When atoms is involved in the formation of covalent compounds?
- Related Articles
How Do Atoms Form Compounds?
When atoms combine through chemical bonding, they form compounds—unique structures composed of two or more atoms. The basic composition of a compound can be indicated using a chemical formula.
How do atoms make compounds?
Atoms combine with each other to form various compounds. The smallest unit of a substance which can exist independently is called a molecule. So, atoms combine with each other to form molecules. These molecules can be formed through either ionic, metallic, covalent or hydrogen bonding.
Why do atoms form compounds?
Atoms form chemical bonds to make their outer electron shells more stable. The type of chemical bond maximizes the stability of the atoms that form it. … Covalent bonds form when sharing atoms results in the highest stability. Other types of bonds besides ionic and covalent chemical bonds exist, too.
How are compounds formed?
A compound is a unique substance that forms when two or more elements combine chemically. Compounds form as a result of chemical reactions. The elements in compounds are held together by chemical bonds. A chemical bond is a force of attraction between atoms or ions that share or transfer valence electrons.
What kind of atoms form compounds?
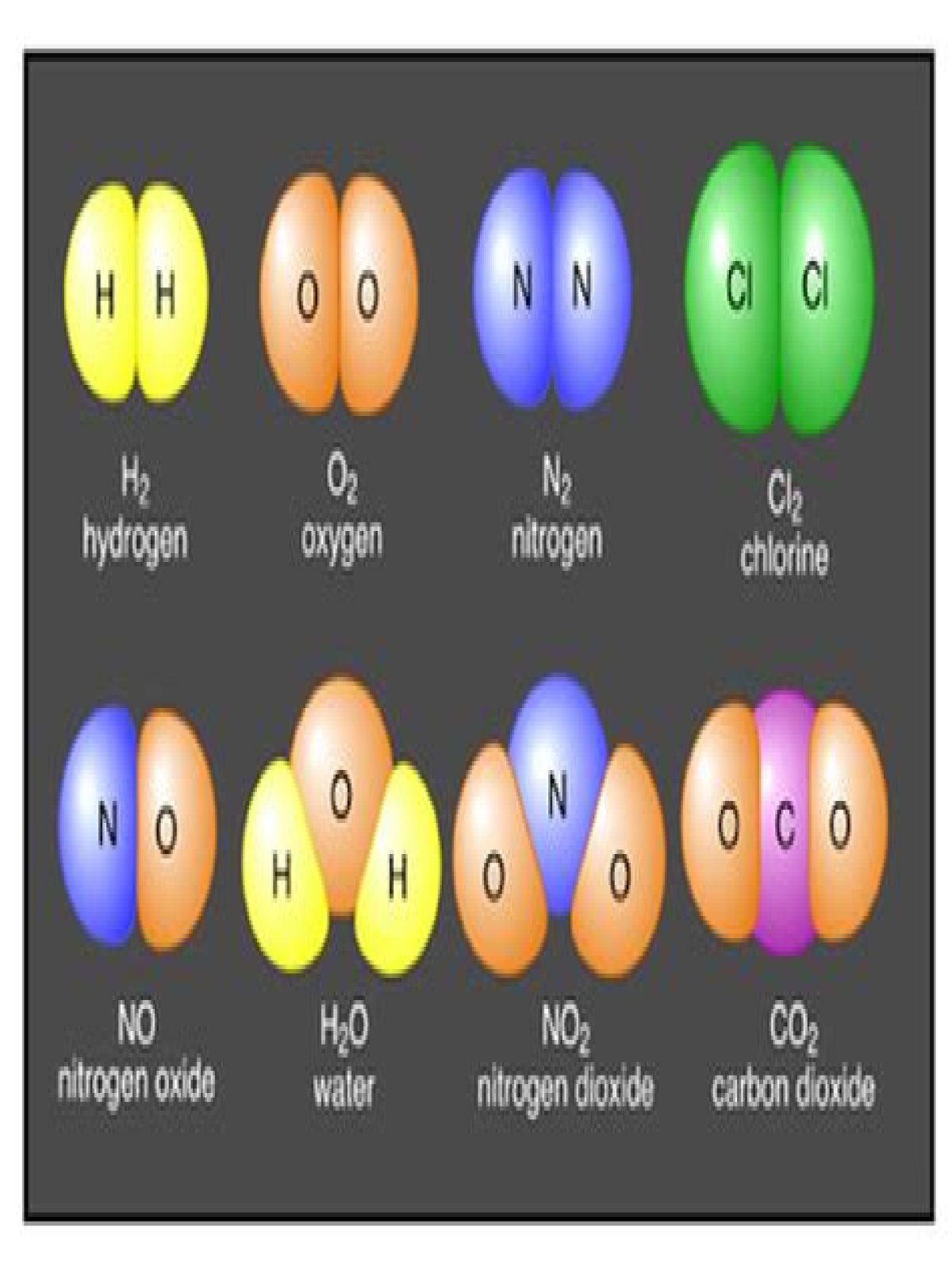
When two or more atoms of different elements join together, we call it a compound. All compounds are molecules, but not all molecules are compounds. That is because a molecule can be made up of two atoms of the same kind, as when two oxygen atoms bind together to make an oxygen molecule.
Does atoms combine to form compounds?
When atoms combine through chemical bonding, they form compounds—unique structures composed of two or more atoms. … Compounds can be covalent or ionic. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. An example of a covalent compound is ammonia.
How do atoms bond with each other to form molecules?
Molecular Formation
Unpaired electrons in the highest energy level are called valence electrons; when the valence electrons from two or more atoms form pairs, they are not lost from one atom and gained by another. The atoms share their valence electrons and bond together, forming a molecule.
Why do atoms form ionic bonds?
Ionic bonds are formed through the exchange of valence electrons between atoms, typically a metal and a nonmetal. The loss or gain of valence electrons allows ions to obey the octet rule and become more stable. Ionic compounds are typically neutral. Therefore, ions combine in ways that neutralize their charges.
How are compounds formed and separated?
When two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. … Water is another example of a chemical compound. The two or more component elements of a compound can be separated through chemical reactions.
What creates an ionic compound?
How are compounds formed 8?
When two elements are combined through a chemical reaction then a compound forms. For example water is a compound formed from the combination of hydrogen and oxygen. 2H2 + O2 → 2H2O.
Why do atoms form compounds quizlet?
Why do atoms form compounds? The electric forces between oppositely charged electrons and protons hold atoms and molecules together, and thus are the forces that cause compounds to form.
When elements react their atoms join with other atoms what type of substance is formed?
When elements react, their atoms join with other atoms to form compounds. Some compounds are made from metals combined with non-metals, for example sodium chloride and magnesium oxide.
What will be produced when two different atoms are combined?
When and where were the first compounds created?
The first organic molecules formed about 4 billion years ago. This may have happened when lightning sparked chemical reactions in Earth’s early atmosphere. RNA may have been the first organic molecule to form as well as the basis of early life.
How does an atom become a positive ion?
The atom that has lost an electron becomes a positively charged ion (called a cation), while the atom that picks up the extra electron becomes a negatively charged ion (called an anion). Opposite charges attract one another while similar charges repel one another.
How are covalent compounds formed?
Covalent Bonds vs Ionic Bonds
| Covalent Bonds | |
|---|---|
| Formation: | A covalent bond is formed between two non-metals that have similar electronegativities. Neither atom is “strong” enough to attract electrons from the other. For stabilization, they share their electrons from outer molecular orbit with others |
| Shape: | Definite shape |
What is formed after an atom gains electrons?
When an atom loses or gains electrons it becomes an ion. The ions are basically of two types, these are cations and anions. Cations are positively charged ions that usually have more protons as compared to electrons. … Therefore the answer is, an ion is formed when an atom loses or gain electrons.
How are compounds formed short answer?
Compounds are formed when two or more different elements react together. These elements become chemically joined and a new substance is formed. This new substance is a compound.
What are the 10 compounds?
Chemical Compound Formulas
| Compound name | Molecular formula | |
|---|---|---|
| 10 | Ammonium sulfate | (NH4)2SO4 |
| 11 | Carbonic acid | H2CO3 |
| 12 | Sodium bicarbonate | NaHCO3 |
| 13 | Sodium hydroxide | NaOH |
What are the two parts of an atom What are they made up of?
How do atoms form chemical bonds quizlet?
Why do most atoms form chemical bonds. They want a full outer shell of electrons, so the lose, gain, or share electrons with other elements, forming compounds, until they have 8 valence electrons and become stable. … An attraction between atoms and/or molecules will lead to chemical bonding.
How is an ionic bond formed quizlet?
ionic bonds form when electrons are transferred from one atom to another atom. ions of different elements can combine by forming ionic bonds . positive ions & negative ions form when atom s lose or gain electrons. … Atoms ,non-metal of elements tend to lose electrons when they form bonds.
How are combinations of different atoms held together?
Every combination of atoms is a molecule. … There are two main types of chemical bonds that hold atoms together: covalent and ionic/electrovalent bonds. Atoms that share electrons in a chemical bond have covalent bonds. An oxygen molecule (O2) is a good example of a molecule with a covalent bond.
How atoms unite and change into molecules?
When atoms join together to form molecules, they are held together by chemical bonds. These bonds form as a result of the sharing or exchange of electrons between the atoms. … Different atoms use these electrons to form one of three different types of bond: ionic bonds, covalent bonds, or metallic bonds.
Can atoms be divided or broken?
Atoms are the smallest possible unit of matter- they can’t be split apart or created or destroyed. We now know that this isn’t true at all- atoms are composed of smaller particles, called protons, neutrons, and electrons.
What is formed when two or more atoms chemically combine?
When two or more atoms chemically bond together, they form a molecule. Sometimes the atoms are all from the same element. For example, when three oxygen atoms bond together, they form a molecule of ozone (O3). If a molecule forms from atoms of two or more different elements, we call it a compound.
How were the first compounds created?
The first organic compounds were formed from the carbon injected into the interstellar medium under the influence of cosmic rays and ultraviolet light. Simple hydrocarbons and other compounds that contain nitrogen, oxygen, and sulfur were formed in this cloud of dust and molecules.
What is the first compound formed?
Scientists finally spied a long-predicted molecule called helium hydride, or HeH+, believed to be the first compound ever formed in the universe.
What was the first atom formed?
It took 380,000 years for electrons to be trapped in orbits around nuclei, forming the first atoms. These were mainly helium and hydrogen, which are still by far the most abundant elements in the universe.